low libido, erectile dysfunction, chronic fatigue
A Few Case Reports
 A 37 year old professional athlete arrived in my office complaining of low testosterone symptoms of low libido, erectile dysfunction, chronic fatigue, and mood disorder. He admitted to anabolic steroid abuse in the past, and now sought medical intervention to "restore his testosterone to normal." A few years ago, he had married and fathered a child, and he now wanted to devote more time to his family, but complained of a lack of energy to do so. He also wanted to preserve fertility, as he wanted more children. Previous medical doctor's lab studies showed low testosterone levels, all below 300 ng/dl, and low FSH and LH levels as well
A 37 year old professional athlete arrived in my office complaining of low testosterone symptoms of low libido, erectile dysfunction, chronic fatigue, and mood disorder. He admitted to anabolic steroid abuse in the past, and now sought medical intervention to "restore his testosterone to normal." A few years ago, he had married and fathered a child, and he now wanted to devote more time to his family, but complained of a lack of energy to do so. He also wanted to preserve fertility, as he wanted more children. Previous medical doctor's lab studies showed low testosterone levels, all below 300 ng/dl, and low FSH and LH levels as well
After our usual workup, and the obvious diagnosis of hyopogonadal hypogonadism, treatment was started with HCG (human chorionic gonadotropin), an LH analog which stimulates testicular testosterone production. The patient wished to retain fertility which contra-indicated the use of Testosterone preparations.
Shortly after starting the HCG injections, the patient reported an immediate improvement in mood and energy, lasting about one week. However, this improvement was short lived and lasted only one week, after which he reported a recurrence of more severe low testosterone symptoms, worse than before.
Repeat labs at 6 weeks showed testosterone levels had actually dropped lower to the 150 ng/dl range. FSH and LH were undetectable. My diagnosis at this point was hypothalamic suppression, and the HCG was discontinued. Treatment with Clomid (clomiphene 25 mg tablet daily) was started. Six weeks later, the patient reported "feeling like my old self" with improved energy, libido and mood. Repeat labs 6 weeks after starting the Clomid showed testosterone levels of 832 ng/dl, and LH and FSH had increased as well. Serum estrogen was quite high at 72 pg/ml. Anastrazole was added to the treatment program with follow up normalization of estrogen levels.
What happened in this case?
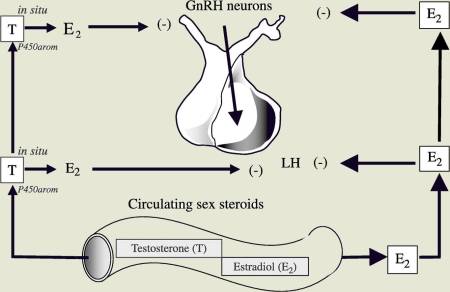 The hypothalamus produces (GnRH), gonadotropin releasing hormone, which in turn stimulates the pituitary production of LH and FSH (leutinizing hormone and follicle stimulating hormone). In the female, estrogen receptors in the hypothalamus represent the "thermostat" control of GnRH and pituitary LH and FSH production.
The hypothalamus produces (GnRH), gonadotropin releasing hormone, which in turn stimulates the pituitary production of LH and FSH (leutinizing hormone and follicle stimulating hormone). In the female, estrogen receptors in the hypothalamus represent the "thermostat" control of GnRH and pituitary LH and FSH production.
Males have this same estrogen receptor in the hypothalamus, and "blocking the estrogen receptor" with a drug such as clomiphene, "tricks" the hypothalamus to produce more GnHR, which in turn increases LH and FSH production by the pituitary.
In this patient, the hypothalamic thermostat was set to shut off at a relatively low estrogen level. In addition, this patient was a "preferential aromatizer.", meaning he had increased conversion of testosterone to estrogen via the aromatase pathway. This explains the paradoxical response to HCG which initially raised testosterone levels, which was then aromatized to estrogen. The increased estrogen levels then shut off the hypothalamic production of GnHR, which in turn caused very low levels of LH and FSH, explaining the low testosterone levels after HCG treatment.
In this scenario, Clomiphene is an excellent treatment, which successfully raises testosterone levels while preserving fertility. Because of preferential conversion of testosterone to estrogen in some men, elevated estrogen levels may require the addition of an aromatase inhibitor such as anastrazole.
Estrogen Feedback Regulates Hypothalamus
In a 2006 report in the European Journal of Endocrinology by Dr Rochira from Italy showed that the feedback of gonadotropins (GnRH from the hypothalamus) is regulated by estrogens that come from the aromatization of testosterone. He studied two males with absent estrgoen from a genetic deficiency in the aromatase enzyme. When these two subjects were given topical Estradiol, this suppressed levels of GnRH, LH, FSH and testosterone, which decreased significantly. (1)
Clomiphene: How does it work ?
Clomiphene binds to and "blocks" the estrogen receptor sites in the hypothalamus (reference). This stimulates the production of GnRH, gonadotropins.
Interesting Medical Findings:
Clomid can cause or exacerbate depression (1)
Clomid increases IGF Binding protein but decreases IGF-1 levels. (2)
Clomid lowers bad cholesterol and raises good cholesterol (3)
Clomid has been shown to increase luteinizing hormone (LH) and follicle stimulating hormone (FSH) (4), which in turn raises testosterone production.(1)
//www.ncbi.nlm.nih.gov/entrez/...2999&query_hl=1
(2)
//www.ncbi.nlm.nih.gov/entrez/...6123&query_hl=3
(3)
//www.ncbi.nlm.nih.gov/entrez/...636&query_hl=10
(4)
//www.ncbi.nlm.nih.gov/entrez/...240&query_hl=21
Impotence Related to Anabolic Steroid Use in a Body Builder
The RECREATIONAL use of anabolic steroids has become commonplace among athletes. Exercise enthusiasts frequently subscribe to information from such sources as the "Underground Steroid Handbook” and self-design illicit drug therapy, including the use of human chorionic gonadotropin (hCG), clomiphene citrate (Clomid), and tamoxifen citrate (Nolvadex), to counter the side effects of gynecomastia and reduced testicular volume.
Despite this apparent drug sophistication, not only can these persons have a psychological dependence on the anabolic steroids, but hypogonadotropic hypogonadism that lasts for months to years may also develop.
The case presented here illustrates the degree of drug knowledge among body builders, the psychosocial dependence on these drugs, and the potential of clomiphene in treating the disorder of pituitary-gonadal failure in such persons.
The patient, a 29-year-old man, had impotence and decreased libido for a year. He is a college student and a competitive body builder who had used anabolic steroids for eight months, alternating 16-week cycles of testosterone cypionate (Depo- Testosterone), 1,500 to 1,800 mg per week, and oxymetholone (Anadrol), 560 mg per week. After stopping the use of these drugs, he was impotent with no spontaneous erections and had diminished libido. He completed a self-selected four-week trial of human chorionic gonadotropin (hCG) without any change in libido and no improvement in potency. The dose of hCG is unknown, and the patient denied any previous use of the drug. He was advised by colleagues to take a course of
clomiphene or await the spontaneous return of sexualfunction. He elected to wait for nine months, without success.
He sought endocrine consultation, almost a full year after his last steroid dose, because of
continued impotence and reduced libido. On examination he was robust, weighing 76 kg (168 lb), height 178 cm (5 ft 10 in), appearing healthy, and was heavily muscled.
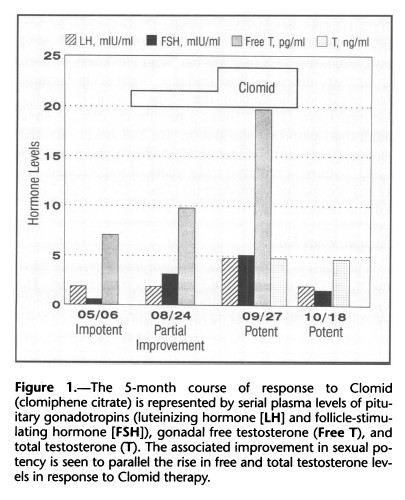 He had a reduced testicular volume of 10 ml on both sides and 2 cm of gynecomastia on both sides. A urine screening test for exogenous anabolic steroids was negative for 19 steroids or metabolites, including danazol, fluoxymesterone, methyltestosterone, 19-nortestosterone, oxymetholone, and stanozolol, as well as the diuretic probenecid. An adrenocorticotropic hormonestimulation test showed a normal rise in the cortisol level from 360 to 830 nmol per liter (13 to 30 ,ug per dl). Magnetic resonance imaging with gadolinium enhancement revealed a normal pituitary gland. Serum gonadotropin and free testosterone levels were abnormal, however, as shown in Figure 1, with a follicle-stimulating hormone (FSH) level of 0.6 mIU per ml (1.6 to 17.8 mIU per ml), a luteinizing hormone (LH) level of 1.9 mIU per ml (1.4 to 11.1 mIU per ml), and a free testosterone level of 7.1 pg per ml (19.0 to 41.0 pg per ml. Treatment was initiated with clomiphene, 50 mg orally per day, and after a month of therapy he had noticed no improvement in potency or libido, although he had begun having morning erections. Serum hormone tests showed moderate improvement in FSH, LH, and free testosterone levels, although not in the normal range (Figure 1). A month after taking a double dose of clomiphene (100 mg per day), the patient reported an increase in libido and potency, and he was able to have sexual intercourse daily. His gonadal volume was unchanged, although serum FSH, LH, and free testosterone levels had reached normal for his age (Figure 1).
He had a reduced testicular volume of 10 ml on both sides and 2 cm of gynecomastia on both sides. A urine screening test for exogenous anabolic steroids was negative for 19 steroids or metabolites, including danazol, fluoxymesterone, methyltestosterone, 19-nortestosterone, oxymetholone, and stanozolol, as well as the diuretic probenecid. An adrenocorticotropic hormonestimulation test showed a normal rise in the cortisol level from 360 to 830 nmol per liter (13 to 30 ,ug per dl). Magnetic resonance imaging with gadolinium enhancement revealed a normal pituitary gland. Serum gonadotropin and free testosterone levels were abnormal, however, as shown in Figure 1, with a follicle-stimulating hormone (FSH) level of 0.6 mIU per ml (1.6 to 17.8 mIU per ml), a luteinizing hormone (LH) level of 1.9 mIU per ml (1.4 to 11.1 mIU per ml), and a free testosterone level of 7.1 pg per ml (19.0 to 41.0 pg per ml. Treatment was initiated with clomiphene, 50 mg orally per day, and after a month of therapy he had noticed no improvement in potency or libido, although he had begun having morning erections. Serum hormone tests showed moderate improvement in FSH, LH, and free testosterone levels, although not in the normal range (Figure 1). A month after taking a double dose of clomiphene (100 mg per day), the patient reported an increase in libido and potency, and he was able to have sexual intercourse daily. His gonadal volume was unchanged, although serum FSH, LH, and free testosterone levels had reached normal for his age (Figure 1).
After clomiphene therapy was discontinued three weeks later, the serum FSH and LH levels fell to normal, and the total serum testosterone remained at a normal level of 16.3 nmol per liter (4.7 ng per ml) (range, 12.5 to 34.5 nmol per liter [3.6 to 9.9 ng per ml]). This response suggested a restoration of normal hypothalamic-pituitarygonadal function, and it was proposed to reevaluate this function with a longer follow-up to determine whether the correction was sustained.
Follow-up of the patient six months later revealed that he had returned to the illicit use of Depo-Testosterone at 400 mg per week to achieve a level of sexual performance three times that achieved with clomiphene alone. He noted that his testes were smaller, and he was considering trying another course of hCG in combination with tamoxifen to prevent worsening gynecomastia.
Discussion
The illicit use of anabolic steroids is becoming more widespread, especially among those involved in competitive athletics or body building and even among teenagers. Even when gonadal dysfunction occurs, persons often continue using the anabolic steroids, in part
because of the neuropsychiatric effects, which include psychotic symptoms, affective syndromes, increased aggression, and psychological dependence. In lay literature, it is common to find medical discussions and advertisements concerning anabolic steroids, androgen supplements, and agents used to combat the side effects of gynecomastia, hirsutism, fluid retention, and acne (MuscleMag International, September 1994, pp 280- 281).
Most synthetic anabolic steroids have some androgenic effects that inhibit gonadotropin-releasing hormone (GnRH) release from the hypothalamus and FSH and LH release from the anterior pituitary. This results in a hypogonadotropic state, and if the agents are used for a prolonged period, testicular atrophy with reduced serum testosterone levels results, causing reduced libido and impotence. When their use is discontinued, the feedback inhibition of GnRH, FSH, and LH synthesis and release is removed and the hypogonadotropic hypogonadism is expected to resolve. According to the literature reports, this usually occurs within four months.
Only two cases have been reported in which suppression of the hypothalamic-pituitary-testicular axis lastedlonger than four months. The first of these patients was administered hCG, and the outcome was determined to be successful when his wife conceived. The second
patient presented with decreased libido three years after his last use of anabolic steroid and was found to have a severely blunted response to a GnRH-stimulation test, consistent with hypothalamic-pituitary suppression.
The patient in the case reported here is unique not only in the year-long suppression of his hypothalamicpituitary- gonadal axis, but also in the successful response to hypothalamic-pituitary stimulation with clomiphene. Although we do not know his gonadotropin and testosterone levels before he began using steroids, it is unlikely he had a preexisting GnRH-deficiency state (such as Kallmann's syndrome) as he had normal secondary sexual development of phallus and hair distribution before initiating exogenous steroid use. We assume that he was compliant in abstaining from exogenous steroids during the treatment period, based on the negative drug screen and compliance with clomiphene administration. More frequent, random screening would be needed to confirm this assumption. The self-administration of hCG should have elicited a testosterone response, but it is uncertain whether he received true hCG in adequate dosage. Because he perceived the failure of a self-initiated hCG trial, we opted for the use of clomiphene at dosages commonly used in women with
hypothalamic-pituitary-ovarian failure. Clomiphene use has previously been reported for the treatment of men who, during evaluation for infertility, are found to have marginal testicular failure or poor gonadotropin production.
Clomiphene appears to produce an antiestrogen effect on the hypothalamus that results in increased GnRH release. In addition, clomiphene exerts an estrogenlike effect on the pituitary, increasing pituitary sensitivity to GnRH.
We propose that with the use of clomiphene we were able to augment the hypothalamic and pituitary responses to his low but not absent ambient estrogen derived by aromatization from testosterone. This is the first reported case of clomiphene-induced restoration of FSH, LH, and free testosterone levels in a man with recreational steroid-induced pituitary-gonadal failure.
******************************************************************************************************************
Clomid quadrupled testosterone level of over-trained runner
A relatively modest dose of clomid – full name clomiphene citrate – quadrupled the amount of testosterone in the body of an endurance athlete, who had wrecked his hormone system by over training. Endocrinologists at the University of New Mexico described what happened in a case study published twelve years ago in Fertility & Sterility.
The combination of endurance sports and over training spells disaster for sex hormone production. One of the most important reasons for this is that over training causes the hypothalamus in the brain to stop producing the master hormone GnRH. GnRH stimulates the production of LH and FSH in the pituitary. These are two hormones that stimulate the Leydig cells to produce more testosterone.
Anti-oestrogens increase the production of GnRH. The more oestrogens there are in the body, the less active the hypothalamus becomes, and the lower the amount of oestrogens, the more active it becomes. An anti-oestrogen like clomid blocks the oestrogen receptor. If you take clomid, oestrogens do continue to circulate in your body, but the cells don't notice them.
The researchers decided to apply this knowledge to a 29-year-old man who showed signs of serious over training. The man was 1.70 metres tall and weighed only 52 kg, but as a result of exercise had developed stress fractures in his pelvis. He had been running between 80 and 140 km a week since he was fifteen. For most people running is good for their bones, but things turned out differently for this guy. He was suffering from osteoporosis.
He’d also had sexual problems since the age of twenty: he’d had increasing trouble getting an erection.
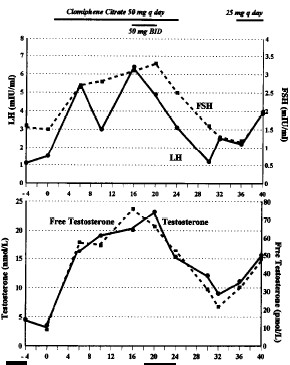 When the doctors tested his blood, they discovered that the man’s testes were producing too little testosterone. His total testosterone level was 4.5 nmol/L. A normal level for men is between 12.5 and 34.3 nmol/L. The man’s free testosterone level was 9.0 pmol/L. The normal level for this is 45.0 to 138.7 pmol/L. The man’s LH and FSH levels were just within the normal limits, but were on the low side.
When the doctors tested his blood, they discovered that the man’s testes were producing too little testosterone. His total testosterone level was 4.5 nmol/L. A normal level for men is between 12.5 and 34.3 nmol/L. The man’s free testosterone level was 9.0 pmol/L. The normal level for this is 45.0 to 138.7 pmol/L. The man’s LH and FSH levels were just within the normal limits, but were on the low side.
The doctors gave the guy 50 mg clomid daily. The graphs below show that as a result his testosterone level rose after week 0 – the start of the clomid therapy – by a factor of four. If you calculate generously it’s a factor of five.
In week 24 the doctors stopped giving the guy clomid. When the complaints returned as a result, and had not disappeared after three months, the doctors put the guy on 25 mg clomid per day. The man apparently was not prepared to change his lifestyle in a way that would normalise his testosterone levels naturally. He could have done this for example by replacing part of his endurance training schedule with strength training, by reducing the total amount of training he did, by sleeping more or by increasing his intake of mono-unsaturated fatty acids.
*******************************************************************************************************************
For the really interested
Eur J Endocrinol. 2006 Oct;155(4):513-22.
Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Rochira V, Zirilli L, Genazzani AD, Balestrieri A, Aranda C, Fabre B, Antunez P, Diazzi C, Carani C, Maffei L.
Source Integrated Department of Medicine, Endocrinology, Metabolism and Geriatrics, University of Modena and Reggio Emilia, Modena, Italy.
BACKGROUND: In men, the feedback of gonadotropins is regulated by estrogens that come from the aromatization of testosterone, but the relative contribution to the inhibition of LH and FSH secretion by the amount of locally produced estrogens within the hypothalamus and/or the pituitary, and the amount of circulating estrogens still remains unknown.
OBJECTIVE:In order to evaluate the effect of regulation induced by estradiol on the hypothalamic-pituitary-gonadal (HPG) axis, we studied the pulsatility of LH and FSH in two aromatase-deficient men (called subject 1 and subject 2), in which the production rate of estrogen (both local and circulating) is completely, or at least severely, impaired.
DESIGN:FSH and LH were evaluated in terms of their pulsated secretion and as GnRH-stimulated secretion in two phases: phase 1, before estrogen treatment; and phase 2, during estrogen treatment with 25 microg transdermal estradiol twice weekly.
METHODS:Blood samples were taken during phase 1 and phase 2 at 0800 h for basal measurements of LH, FSH, inhibin B, testosterone, and estradiol. The analysis of the pulsatility of LH and FSH was performed by sampling every 10 min for 8 h in the two phases. Gonadotropin response to GnRH-stimulation test was studied by serial standard sampling after 100 microg GnRH i.v. bolus in phases 1 and 2.
RESULTS:Estrogen treatment led to a significant reduction in both LH-pulsated frequency (7.5 +/- 0.7 in phase 1, 4.5 +/- 0.7 in phase 2) and amplitudes (3.5 +/- 0.006 in phase 1, 1.9 +/- 0.4 in phase 2) of peaks, whereas FSH showed only a conspicuous reduction in serum levels and a trend towards the reduction of the amplitudes of its peaks without modification of the frequency of the pulses.
Both testosterone and gonadotropins decreased during phase 2, whereas estradiol reached the normal range in both subjects.
Transdermal estradiol treatment significantly lowered the peaks of both serum LH and FSH after GnRH as well as the incremental area under the curve after GnRH administration in both subjects. Basal serum inhibin B levels were slightly higher before transdermal estradiol treatment (phase 1) than during estrogen treatment (phase 2) in both subjects.
CONCLUSIONS:The administration of estrogen to aromatase-deficient men discloses the effects of circulating estrogens on LH secretion, exerted both at pituitary level, as shown by the decrease of basal and GnRH-stimulated secretion of LH and the LH pulsed amplitude, and at hypothalamic level as shown by the reduction of the frequency of LH pulses. The present study, coupling the outcomes of basal, GnRH-stimulated and the pulsatile evaluation of LH and FSH secretion in two aromatase-deficient men, demonstrates that circulating estrogens play an inhibitory role in LH secretion by acting on the hypothalamus and the pituitary gland of men. The discrepancy among testosterone levels, the arrest of spermatogenesis and a slightly inappropriate respective increase of serum FSH (lower than expected) suggests a possible role of estrogens in the priming and the maturation of HPG axis in men, an event that has never occurred in these two subjects as a consequence of chronic estrogen deprivation.
2012
www.ncbi.nlm.nih.gov/pubmed/22458540
BJU Int. 2012 Mar 28.
Clomiphene citrate is safe and effective for long-term management of hypogonadism.
Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP.Sexual & Reproductive Medicine Program, Urology Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Clomiphene citrate (CC) has previously been documented to be efficacious in the treatment of hypogonadism. However little is known about the long term efficacy and safety of CC. Our study demonstrates that CC is efficacious after 3 years of therapy. Testosterone levels and bone mineral density measurement improved significantly and were sustained over this prolonged period. Subjective improvements were also demonstrated. No adverse events were reported.
OBJECTIVE: To assess the efficacy and safety of long-term clomiphene citrate (CC) therapy in symptomatic patients with hypogonadism (HG).
PATIENTS AND METHODS:Serum T, oestradiol and luteinizing hormone (LH) were measured in patients who were treated with CC for over 12 months. • Additionally, bone densitometry (BD) results were collected for all patients. Demographic, comorbidity, treatment and Androgen Deficiency in Aging Men (ADAM) score data were also recorded.
• Comparison was made between baseline and post-treatment variables, and multivariable analysis was conducted to define predictors of successful response to CC.
• The main outcome measures were predictors of response and long-term results with long-term CC therapy in hypogonadal patients. RESULTS:The 46 patients (mean age 44 years) had baseline serum testosterone (T) levels of 228 ng/dL.
• Follow-up T levels were 612 ng/dL at 1 year, 562 ng/dL at 2 years, and 582 ng/dL at 3 years (P < 0.001).
• Mean femoral neck and lumbar spine BD scores improved significantly.
• ADAM scores (and responses) fell from a baseline of 7 to a nadir of 3 after 1 year. • No adverse events were reported by any patients. CONCLUSIONS:Clomiphene citrate is an effective long-term therapy for HG in appropriate patients.
• The drug raises T levels substantially in addition to improving other manifestations of HG such as osteopenia/osteoporosis and ADAM symptoms.
--------------------------------------------2011
www.ncbi.nlm.nih.gov/pubmed/22044663
BJU Int. 2011 Nov 1.
Outcomes of clomiphene citrate treatment in young hypogonadal men.Katz DJ, Nabulsi O, Tal R, Mulhall JP.
Source Male Sexual and Reproductive Medicine Programme, Urology Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Study Type - Therapy (case series) Level of Evidence 4 What's known on the subject? and What does the study add? Hypogonadism is a prevalent problem, increasing in frequency as men age. It is most commonly treated by testosterone supplementation therapy but in younger patients this can lead to testicular atrophy with subsequent exogenous testosterone dependency and may impair spermatogenesis.
Clomiphene citrate (CC) may be used as an alternative treatment in these patients with hypogonadism when maintenance of fertility is desired. This study shows that CC is a safe and efficacious drug to use as an alternative to exogenous testosterone. Not only have we validated previous findings of other papers but have proven our findings over a much longer period (mean duration of treatment 19 months). This prospective study is the largest to date assessing both the objective hormone response to CC therapy as well as the subjective response based on a validated questionnaire.
OBJECTIVE:To prospectively assess the andrological outcomes of long-term clomiphene citrate (CC) treatment in hypogonadal men.
PATIENTS AND METHODS:We prospectively evaluated 86 men with hypogonadism (HG) as confirmed by two consecutive early morning testosterone measurements <300 ng/dL.
• The cohort included all men with HG presenting to our clinic between 2002 and 2006 who, after an informed discussion, elected to have CC therapy. CC was commenced at 25 mg every other day and titrated to 50 mg every other day.
The target testosterone level was 550 ± 50 ng/dL.
• Testosterone (free and total), sex hormone binding globulin, oestradiol, luteinizing hormone and follicle stimulating hormone were measured at baseline and during treatment on all patients. Once the desired testosterone level was achieved, testosterone/gonadotropin levels were measured twice per year.
• To assess subjective response to treatment, the androgen deficiency in aging males (ADAM) questionnaire was administered before treatment and during follow-up.
RESULTS: patients' mean (standard deviation [sd]; range) age was 29 (3; 22-37) years. Infertility was the most common reason (64%) for seeking treatment. The mean (sd) duration of CC treatment was 19 (14) months.
• At the last evaluation, 70% of men were using 25 mg CC every other day, and the remainder were using 50 mg every other day.
• All mean testosterone and gonadotropin measurements significantly increased during treatment.
• Subjectively, there was an improvement in all questions (except loss of height) on the ADAM questionnaire. More than half the patients had an improvement in at least three symptoms.
• There were no major side effects recorded and the presence of a varicocele did not have an impact on the response to CC.
CONCLUSION:Long-term follow-up of CC treatment for HG shows that it appears to be an effective and safe alternative to testosterone supplementation in men wishing to preserve their fertility
--------------------------------------------2010
www.ncbi.nlm.nih.gov/pubmed/19694928
J Sex Med. 2010 Jan;7(1 Pt 1):269-76. Epub 2009 Aug 17.
Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost.
Taylor F, Levine L. Rush University Medical Center-Department of Urology, Chicago, IL, USA.
INTRODUCTION:The efficacy of oral clomiphene citrate (CC) in the treatment of male hypogonadism and male infertility (MI) with low serum testosterone and normal gonadotropin levels has been reported.
AIM:The aim of this article is to evaluate CC and testosterone gel replacement therapy (TGRT) with regard to biochemical and clinical efficacy and cost. MAIN OUTCOME MEASURES:The main outcome measures were change in serum testosterone with CC and TGRT therapy, and change in the androgen deficiency in aging male (ADAM) questionnaire scores with CC therapy.
METHODS:Men receiving CC or TGRT with either Androgel 1% or Testim 1% for hypogonadism (defined as testosterone < 300 ng/mL) or MI were included. Serum values were collected 1-2 months after treatment initiation and semi-annually thereafter. Retrospective data collection was performed via chart review. Subjective follow up of patients receiving CC was performed via telephone interview using the ADAM questionnaire.
RESULTS:A hundred and four men (65 CC and 39 TGRT) were identified who began CC (50 mg every other day) or TGRT (5 g). Average age (years) was 42(CC) vs. 57 (TGRT). Average follow up was 23 months (CC, range 8-40 months) vs. 46 months (TGRT, range 6-149 months).
'Average posttreatment testosterone was 573 ng/dL in the CC group and 553 ng/dL in the TGRT group (P value < 0.001). The monthly cost of Testim 1% (5 gm daily) is $270, Androgel 1% (5 gm daily) is $265, and CC (50 mg every other day) is $83. Among CC patients, the average pretreatment ADAM score was 4.9 vs. 2.1 at follow up (P < 0.05). Average pretreatment ADAM sexual function domain score was 0.76 vs. 0.23 at follow up (P < 0.05). There were no adverse events reported.
CONCLUSION:CC represents a treatment option for men with hypogonadism, demonstrating biochemical and clinical efficacy with few side effects and lower cost as compared with TGRT.
---------------------------------------------------------------------------2011
jcem.endojournals.org/content/96/1/38.full#ref-28
The Journal of Clinical Endocrinology & Metabolism January 1, 2011 vol. 96 no. 1 38-52
Why Is Androgen Replacement in Males Controversial? Glenn R. Cunningham and Shivani M. Toma Baylor College of Medicine and St. Luke’s Episcopal Hospital, Houston, Texas 77030 2009
--------------------------------------------2009
www.ncbi.nlm.nih.gov/pubmed/19204885
IDrugs. 2009 Feb;12(2):109-19.
Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men.
Hill S, Arutchelvam V, Quinton R. Department of Clinical Pharmacology & Therapeutics, Royal Victoria Infirmary, Queen Victoria Road, Newcastle upon Tyne, NE1 4LP, UK.
Enclomiphene (Androxal), in development by Repros Therapeutics Inc, is a non-steroidal estrogen receptor antagonist that promotes gonadotropin-dependent testosterone secretion by the testes. Enclomiphene constitutes the trans-stereoisomer of clomiphene citrate, a drug that has been widely prescribed for several decades for the treatment of female ovulatory dysfunction. Because of the antagonistic effects of enclomiphene, the drug has the potential to increase serum testosterone levels in men with secondary hypogonadism by restoring physiological endogenous testosterone secretion while maintaining testicular volume and, potentially, spermatogenesis. In clinical trials conducted to date, enclomiphene demonstrated significant efficacy in the physiological restoration of testosterone levels in males with secondary hypogonadism. The compound also exhibited an unanticipated favorable effect on fasting plasma glucose; this result has been accompanied by rapidly accumulating evidence from other researchers for a bidirectional relationship between low serum testosterone and obesity/metabolic syndrome (syndrome X) in men. Short-term clinical safety data for enclomiphene have been satisfactory and equivalent to safety data for testosterone gels and placebo.
Enclomiphene demonstrates promise in the management of secondary hypogonadism associated with obesity, metabolic syndrome and, possibly, infertility, and should undergo placebo-controlled, randomized clinical trials for these indications.
--------------------------------------------2009
www.ncbi.nlm.nih.gov/pubmed/19938905
Expert Opin Investig Drugs. 2009 Dec;18(12):1947-55.
Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency.
Kaminetsky J, Hemani ML. NYU Langone Medical Center - Department of Urology, New York, New York 10016, USA.
Hypogonadism has a number of important clinical consequences related to androgen deficiency and impaired spermatogenesis. The cause of this condition is multifactorial and can result from hypothalamic, pituitary or gonadal dysfunction as well as factors that affect hormonal signaling along the hypothalamic-pituitary-gonadal axis. While testosterone replacement is the most common treatment, it can paradoxically lead to infertility, and may be a less physiologic therapy for patients with secondary hypogonadism due to pituitary dysfunction. Clomiphene citrate, and its derivatives, may allow for restoration of gonadal function by restoring physiologic pituitary function in a subset of patients with hypogonadism.
--------------------------------------------2006
www.ncbi.nlm.nih.gov/pubmed/17070201
Fertil Steril. 2006 Nov;86(5):1513.e5-9.
Complete reversal of adult-onset isolated hypogonadotropic hypogonadism with clomiphene citrate.
Ioannidou-Kadis S, Wright PJ, Neely RD, Quinton R. Department of Endocrinology, Royal Victoria Infirmary and University of Newcastle-upon-Tyne, Newcastle-upon-Tyne, United Kingdom.
Inhibition of pituitary gonadotropin secretion in men by T is principally mediated by aromatization to estrogen (E), which inhibits hypothalamic secretion of GnRH. We hypothesized that adult-onset isolated hypogonadotropic hypogonadism (IHH) might result from an altered central set-point for E-mediated negative feedback. Longitudinal clinical investigation unit-based evaluation of the clinical and biochemical response to E-receptor blockade.
A 31-year-old man presenting with an 18-month history of sexual dysfunction resulting from severe adult-onset IHH (LH 1.7 U/L, FSH 2.0 U/L, T 3.5 nmol/L). Initial therapy with 50 mg of clomiphene citrate (CC) three times a day for 7 days, with overnight LH pulse profiling and 9 am T levels evaluated at baseline and on completion. A 2-month washout period, followed by low-dose maintenance therapy (25-50 mg/d) for 4 months.
MAIN OUTCOME MEASURE(S):Baseline and stimulated T levels and LH pulsatility; effect on sexual function.
RESULT(S):Clomiphene therapy resulted in complete normalization of pulsatile gonadotropin secretion, serum T level, and sexual function. CONCLUSION(S):Isolated hypogonadotropic hypogonadism may result from an acquired defect of enhanced hypothalamic sensitivity to E-mediated negative feedback. Whereas direct T replacement therapy can further suppress endogenous gonadotropin secretion, treating IHH men with gonadotropins can stimulate endogenous T secretion and enhance fertility potential. On theoretical grounds, reversal of gonadotropin deficiency with CC might be expected to have a similar biological effect.
--------------------------------------------2006
www.ncbi.nlm.nih.gov/pubmed/17007848
Fertil Steril. 2006 Dec;86(6):1664-8. Epub 2006 Sep 27.
Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate.
Whitten SJ, Nangia AK, Kolettis PN. Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Alabama, Birmingham, Alabama 35249-7333, USA.
OBJECTIVE: To review the management of male hypogonadotropic hypogonadism (HH) and evaluate the efficacy of clomiphene citrate (CC). DESIGN:Retrospective review.
SETTING:Two university-based urology clinics.
PATIENT(S):Ten patients referred for male infertility evaluation.
INTERVENTION(S): Patients were treated with either clomiphene citrate or injectable gonadotropins.
MAIN OUTCOME MEASURE(S): Changes in seminal parameters, gonadotropin levels, serum testosterone, and pregnancy.
RESULT(S): Ten men who were evaluated for infertility were diagnosed with HH. Four had Kallmann's syndrome, four idiopathic HH, and two panhypopituitarism. Eight patients were azoospermic, and two were oligospermic on presentation. Three of the four men with adult-onset idiopathic HH responded to CC alone with increases in testosterone, FSH, and LH. Semen parameters in this group also improved, and two of the three men achieved pregnancies with CC alone. Out of the ten men actively attempting conception, four pregnancies were achieved. Three pregnancies (two with CC and one with gonadotropins) were in men diagnosed with adult-onset idiopathic forms of HH.
CONCLUSION( S): Select patients with adult-onset idiopathic forms of HH may benefit from a trial of clomiphene citrate.
--------------------------------------------------------
2003 Clomid Restores T after steroid abuse
www.ncbi.nlm.nih.gov/pubmed/12524089
Fertil Steril. 2003 Jan;79(1):203-5.
Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse.
Tan RS, Vasudevan D. Department of Family and Community Medicine, University of Texas Health Sciences Center, Houston, Texas 77030, USA. robert.s.tan@uth.tmc.edu To report a case of symptomatic hypogonadism induced by the abuse of multiple steroid preparations that was subsequently reversed by clomiphene. DESIGN: Case report.
SETTING:University-affiliated andrology practice within family practice clinic.
PATIENT(S):A 30-year-old male.
INTERVENTION(S): Clomiphene citrate, 100-mg challenge for 5 days, followed by treatment at same dose for 2 months.
MAIN OUTCOME MEASURE(S): Clinical symptoms, androgen decline in aging male questionnaire, total T, FSH, LH.
RESULT(S): Reversal of symptoms, normalization of T levels with LH surge, restoration of pituitary-gonadal axis.
CONCLUSION(S): Clomiphene citrate is used typically in helping to restore fertility in females. This represents the first case report of the successful use of clomiphene to restore T levels and the pituitary-gonadal axis in a male patient. The axis was previously shut off with multiple anabolic steroid abuse.
--------------------------------------------1997
www.ncbi.nlm.nih.gov/pubmed/9093212
Fertil Steril. 1997 Apr;67(4):783-5.
Idiopathic hypogonadotropic hypogonadism in a male runner is reversed by clomiphene citrate.
Burge MR, Lanzi RA, Skarda ST, Eaton RP. University of New Mexico School of Medicine, Department of Medicine/Endocrinology-5ACC, Albuquerque 87131, USA.
To assess the efficacy of estrogen antagonist therapy on the function of the hypothalamic-pituitary-testicular axis in a young male runner with significant morbidity attributable to idiopathic hypogonadotropic hypogonadism.
DESIGN: An uncontrolled case study.
SETTING: The outpatient endocrinology clinic of a university tertiary referral center. PATIENT(S): A 29-year-old male who has run 50 to 90 miles per week since 15 years of age and who presented with a pelvic stress fracture, markedly decreased bone mineral density, and symptomatic hypogonadotropic hypogonadism.
INTERVENTION(S): Clomiphene citrate (CC) at doses up to 50 mg two times per day over a 5-month period.
MAIN OUTCOME MEASURE(S): Serum concentrations of LH, FSH, and T before and after CC therapy, as well as clinical indicators of gonadal function.
RESULT(S): Barely detectable levels of LH and FSH associated with hypogonadal levels of T were restored to the normal range with CC therapy.
The patient experienced improved erectile function, increased testicular size and sexual hair growth, and an improved sense of well being.
CONCLUSION(S): Exercise-induced hypogonadotropic hypogonadism exists as a clinical entity among male endurance athletes, and CC may provide a safe and effective treatment option for males with debilitating hypogonadism related to endurance exercise.
-------------------------------------------------------------------------2005
www.ncbi.nlm.nih.gov/pubmed/16422830
J Sex Med. 2005 Sep;2(5):716-21.
Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism.
Shabsigh A, Kang Y, Shabsign R, Gonzalez M, Liberson G, Fisch H, Goluboff E. Department of Urology, NY Presbyterian Medical Center, New York, NY, USA.
Symptomatic late-onset hypogonadism is associated not only with a decline in serum testosterone, but also with a rise in serum estradiol.
These endocrine changes negatively affect libido, sexual function, mood, behavior, lean body mass, and bone density. Currently, the most common treatment is exogenous testosterone therapy. This treatment can be associated with skin irritation, gynecomastia, nipple tenderness, testicular atrophy, and decline in sperm counts.
In this study we investigated the efficacy of clomiphene citrate in the treatment of hypogonadism with the objectives of raising endogenous serum testosterone (T) and improving the testosterone/estrogen (T/E) ratio.
METHODS: Our cohort consisted of 36 Caucasian men with hypogonadism defined as serum testosterone level less than 300 ng/dL. Each patient was treated with a daily dose of 25 mg clomiphene citrate and followed prospectively. Analysis of baseline and follow-up serum levels of testosterone and estradiol levels were performed.
RESULTS: The mean age was 39 years, and the mean pretreatment testosterone and estrogen levels were 247.6 +/- 39.8 ng/dL and 32.3 +/- 10.9, respectively. By the first follow-up visit (4-6 weeks), the mean testosterone level rose to 610.0 +/- 178.6 ng/dL (P < 0.00001). Moreover, the T/E ratio improved from 8.7 to 14.2 (P < 0.001). There were no side effects reported by the patients.
CONCLUSIONS: Low dose clomiphene citrate is effective in elevating serum testosterone levels and improving the testosterone/estradiol ratio in men with hypogonadism. This therapy represents an alternative to testosterone therapy by stimulating the endogenous androgen production pathway.
--------------------------------------------2003
www.ncbi.nlm.nih.gov/pubmed/12904801?dopt=Abstract
Int J Impot Res. 2003 Jun;15(3):156-65.
Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit?
Guay AT, Jacobson J, Perez JB, Hodge MB, Velasquez E. Center for Sexual Function (Endocrinology), Peabody, Massachusetts 01960, USA.
Secondary hypogonadism is more common than primary gonadal failure and is seen in chronic and acute illnesses. Although testosterone has a role in erections, its importance in erectile dysfunction (ED) has been controversial. Hypogonadism produced by functional suppression of pituitary gonadotropins has been shown to correct with clomiphene citrate, but with a modest effect on sexual function. We wondered if longer treatment would produce improved results.
A total of 178 men with secondary hypogonadism and ED received clomiphene citrate for 4 months. Sexual function improved in 75%, with no change in 25%, while significant increases in luteinizing hormone (P<0.001) and free testosterone (P<0.001) occurred in all patients.
Multivariable analysis showed that responses decreased significantly with aging (P<0.05). Decreased responses also occurred in men with diabetes, hypertension, coronary artery disease, and multiple medication use. Since these conditions are more prevalent with aging, chronic disease may be a more important determinant of sexual dysfunction. Men with anxiety-related disorders responded better to normalization of testosterone. Assessment of androgen status should be accomplished in all men with ED. For those with lower than normal age-matched levels of testosterone treatment directed at normalizing testosterone with clomiphene citrate is a viable alternative to giving androgen supplements.
--------------------------------------------1995
www.ncbi.nlm.nih.gov/pmc/articles/PMC1022657/?page=1
West J Med. 1995 February; 162(2): 158–160.
Impotence related to anabolic steroid use in a body builder.
Response to clomiphene citrate. C Bickelman, L Ferries, and R P Eaton Department of Medicine, University of New Mexico School of Medicine, Albuquerque, USA.
--------------------1998 HCG
HCG for reversing anabolic steroid abuse
www.ncbi.nlm.nih.gov/pmc/articles/PMC2360778/?tool=pubmed
Postgrad Med J. 1998 Jan;74(867):45-6.
Anabolic steroid induced hypogonadism treated with human chorionic gonadotropin. Gill GV.Endocrine Unit, Walton Hospital, Liverpool, UK.
A case is presented of a young competitive body-builder who abused anabolic steroid drugs and developed profound symptomatic hypogonadotrophic hypogonadism. With the help of prescribed testosterone (Sustanon) he stopped taking anabolic drugs, and later stopped Sustanon also. Hypogonadism returned, but was successfully treated with weekly injections of human chorionic gonadotropin for three months. Testicular function remained normal thereafter on no treatment. The use of human chorionic gonadotropin should be considered in prolonged hypogonadotrophic hypogonadism due to anabolic steroid abuse.
2005 HCG and HMG
www.ncbi.nlm.nih.gov/pubmed/16316564
Zhonghua Nei Ke Za Zhi. 2005 Nov;44(11):836-9.
[The application of gonadotropin in treatment of male central hypogonadism]. [Article in Chinese] Di FS, Cui YG, Jia Y. Department of Endocrinology and Key Laboratory, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China.
To observe the efficacy of human chorionic gonadotrophin (hCG) and hCG plus human menopausal gonadotropin (HMG) for central hypogonadism in male patients.
64 men with central hypogonadism were recruited in this study, including 19 patients with Kallmann syndrome, 41 patients with idiopathic hypogonadotrophic hypogonadism (IHH) and 4 patients with hypogonadism after brain surgery. 33 patients were treated with hCG 1500 IU intramuscularly twice a week, whereas 31 patients were treated with intramuscular hCG 1500 IU plus HMG 75 IU twice a week, for at least 6 months.
After treatment, all patients felt stronger physically and 42/64 patients developed beard, pubes or armpit hair. The testis volume enlarged significantly [(3.08 +/- 2.44) ml vs (8.92 +/- 5.37) ml, P < 0.001], and serum follicle-stimulating hormone, luteinizing hormone and testosterone concentrations were higher significantly than those before treatment (P < 0.05). 6/64 patients underwent spermatorrhea and 2 patient were found to have spermatogenesis. If judged by the testis volume, 52 patients (81.2%) were effective and 12 patients were ineffective.
CONCLUSIONS:For male patients with the central hypogonadism, hCG and hCG plus HMG can promote the pubertal development and maturation of second sex characteristics, as well as enhance the physical strength; in some patients both androgen production and spermatogenesis can be achieved.
www.ncbi.nlm.nih.gov/pubmed/8009475
Tidsskr Nor Laegeforen. 1994 Feb 10;114(4):426-8.
[Endocrine effects of doping with androgenic anabolic steroids]. [
Article in Norwegian] Birkeland KI, Jørgensen J, Hemmersbach P. Hormonlaboratoriet Aker sykehus, Oslo.
We describe two case histories that highlight some of the endocrine effects of doping with androgenic anabolic steroids. The main endocrine effect observed after use of androgenic anabolic steroids is the development of hypogonadotrope hypogonadism, characterized by low levels of gonadotrophins, suppression of testosterone production and azoospermia.
If testosterone is used alone, or in combination with synthetic anabolic steroids, the circulating levels of testosterone are normal or high. Oestrogen levels may be elevated owing to aromatization of testosterone. The level of sex hormone binding globulin is suppressed. These endocrine parameters are of practical use in evaluating patients misusing androgenic anabolic steroids.
www.jstage.jst.go.jp/article/endocrj/advpub/0/0702070033/_pdf
Endocr J. 2007 Apr;54(2):177-90. Epub 2007 Feb 8.
Management of male hypogonadotrophic hypogonadism.
Howles CM, Tanaka T, Matsuda T. Department of Global Product Development, Merck Serono International SA, Geneva Switzerland.
---------------------------------
www.ncbi.nlm.nih.gov/pubmed/6435491
Ann Intern Med. 1984 Nov;101(5):629-32.
Hypogonadism in hemochromatosis: reversal with iron depletion.
Kelly TM, Edwards CQ, Meikle AW, Kushner JP. Abstract \\
Gonadal function was evaluated in 64 persons homozygous for the HLA-linked hemochromatosis allele. Of 41 men, 10 had reduced libido or impotence and 6 had testicular atrophy. Before treatment, 5 men had below normal testosterone concentrations, 4 of whom also had low gonadotrophin levels. Four hypogonadal men were reevaluated after iron depletion treatment. In 2, 1 with primary and another with secondary hypogonadism, testosterone levels returned to normal after phlebotomy and were accompanied by a return of normal sexual function. None of 23 women with hemochromatosis had loss of libido or had a natural menopause before age 45. Our findings indicate that in some men with hereditary hemochromatosis and hypogonadism of either testicular or central origin, sexual function and sex hormone concentrations can be restored to normal after iron depletion therapy.
----------------------------------------1995
Int J Fertil Menopausal Stud. 1995 Jul-Aug;40(4):187-91.
Nonbacterial pyospermia: a consequence of clomiphene citrate therapy. Matthews GJ, Goldstein M, Henry JM, Schlegel PN. James Buchanan Brady Foundation, Department of Urology, New York Hospital-Cornell University Medical Center, New York, USA.
Since the development of nonbacterial pyospermia in previously nonpyospermic men treated with clomiphene citrate (CC) has been observed, and nonbacterial prostatitis has been after antiestrogen treatment in an animal model, we sought characterize the occurrence of nonbacterial pyospermia in men treated with CC.
PATIENTS AND METHODS: Forty-two nonpyospermic men with low serum testosterone levels treated with 25 mg CC/day were retrospectively compared to 27 untreated nonpyospermic men referred for infertility evaluation.
RESULTS: Spontaneous nonbacterial pyospermia developed in CC-treated men [14.3%] at rate nearly twice that observed in controls [7.4%]. Serum testosterone increased in CC-treated men, both pyospermic and nonpyospermic. However, only CC-treated, nonpyospermic men demonstrated improvement in semen characteristics. CC-treated men who developed pyospermia were older than nonpyospermic men [pyospermic, 41.7 +/- 8.1 years; nonpyospermic, 35.6 +/- 4.9 years-P < .01).
Men over 35 years of age were over six times as likely to develop pyospermia as men under 35 years of age (P < .05). Eight nonpyospermic, CC-treated men (8/36, 22.2%) have contributed to pregnancies leading to live births, whereas no pyospermic man has done so.
CONCLUSION: These findings support an association between a nonbacterial inflammatory response of the human male reproductive tract and CC treatment. This pyospermia may occur without significant deterioration of semen characteristics and with an appropriate response to treatment in terms of serum testosterone level. Men over the age of 35 are statistically more likely to develop pyospermia with this therapy. Our results suggest that clomiphene citrate-associated pyospermia has a negative effect on male fertility.
- Login to post comments


