Growth hormone secratogogues ( specifically MK-0677)

Reading up on this subject I found an older article written by J Emanuelson. It expresses what many of us think. “In a free market, MK-0677 (ibutamoren mesylate) would likely have had a revolutionary impact on the health of most people over 40. In fact, it is possible that MK-0677 could have revolutionized health care, prevented great human suffering, and literally saved trillions of dollars in health care. Since a free market in pharmaceuticals does not exist, MK-0677 will probably remain a laboratory curiosity for many years”
“Even though compounds of the growth hormone secretagogue (GHS) family have all been researched and found effective, it is doubtful if the research will proceed much farther. Most of these compounds are mainly effective against age-related declines in growth hormone. The United States Food and Drug Administration (FDA) does not regard the age-related decline in growth hormone to be a disease, even when it results in serious disability and death. Because of the size of the U.S. market and the worldwide influence of the FDA, these valuable medicines will probably be forever blocked from the market. (Because of the effectiveness of MK-0677, however, there is some hope for it eventually being approved somewhere in the world for a condition other than age-related growth hormone decline.) “
“The increasing oppressiveness of the FDA is causing a backlash against that agency. Many people within the FDA have differing interpretations of their own rules. Do not underestimate the oppressiveness of the FDA. The reach of the FDA is worldwide, and if you run afoul of their regulations, you may find yourself with automatic weapons aimed at your head.”
Of course Growth hormone secratogogues have their place not only in anti-aging but also in the doping and bodybuilding scene. Bodybuilders are used to the fact that almost everything they use to enhance their appearance is prohibited. This made use very inventive and we don’t have to rely on the FDA approved drugs.
Pharmaceutical companies have produced growth hormone releasing agents that have been shown to be very effective in reversing the decline in HGH production with age. The one that has consistently worked the best is MK-0677 (ibutamoren mesylate), which is very effective in restoring HGH release in middle-aged and "normally-aging" elderly individuals to the levels of much younger people. MK-0677 is an oral medicine that restores the release of HGH in the pulsatile fashion characteristic of HGH release in young people. Unfortunately, it was not very effective in restoring HGH in the frail elderly, which was its original target market. It appears, in fact, that any form of HGH supplementation in the very frail elderly, and in the critically-ill elderly, is actually quite harmful. Restoring HGH in "normally-aging" people is not a function that the Food and Drug Administration (FDA) considers to be a legitimate function of a medicine; therefore, Merck (the pharmaceutical company) stopped all further development of MK-0677. Other effective oral HGH releasers developed by the pharmaceutical companies have faced a similar fate for similar reasons.
A considerable amount of research has been done on HGH releasers by the pharmaceutical companies, and some very promising substances have been developed, but there is no sign that any of them will be on the market anytime soon. MK-0677 (ibutamoren mesylate) is a substance, though, that seems to be too good to go away.
Why restrict these compounds?
It’s quite obvious that Big Pharma has no interest at all in people that cure themselves. With the help of staggering profits and 1100-plus paid lobbyists, the industry has gained powerful leverage on Capitol Hill. From 1998 to 2013, Big Pharma spent nearly $2.7 billion on lobbying expenses and the industry have doled out $150 million in campaign contributions.
The world’s 11 largest drug companies made a net profit of $711.4 billion from 2003 to 2012. Six of these companies are headquartered in the United Sates. In 2012 alone, the top 11 companies earned nearly $85 billion in net profits. According to IMS Health, the global market for pharmaceuticals is expected to top $1 trillion in sales by 2014. Big Pharma has a lot of employees and pays a lot of taxes. You get the picture …right?
Endogenous vs exogenous GH
To make the HGH situation even more complex, HGH is normally released in pulses or bursts throughout the day. There are usually 10 to 20 surges of HGH release, with the largest release occurring shortly after you fall asleep. Is there any advantage to having HGH released in pulses? Or is this simply the body's most efficient way of producing HGH? There seems to be some evidence that the pulsatile release of HGH is important for human health.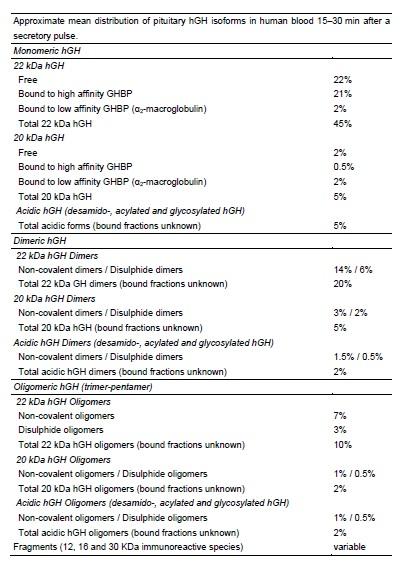
There are indications, however, that some of the ghrelin analogs or the GHRH analogs may be superior to ordinary HGH replacement. Ordinary HGH therapy does not increase insulin sensitivity or decrease glucose levels, although it logically should be expected to -- since it increases the level of IGF-1 (insulin-like growth factor number 1). IGF-1 decreases glucose levels, so there is something about the continuous presence of growth hormone that is offsetting this IGF-1 related decrease in blood glucose. When youthful pulsatile release of growth hormone is restored (with a growth hormone secratogogue), often the IGF-1 related decrease in blood glucose is seen in most people, as would be expected. With some people, however, blood glucose levels increase (at least in short-term studies).
Human growth hormone (GH) exists in a variety of isoforms in the body. In the pituitary, the most abundant isoform is 22-kD GH (22 K GH), while other isoforms (non-22 K GH) are present in variable amounts. In human plasma, the GH heterogeneity contributes to the wide variability in GH levels measured by different immunoassays. The physiological role of the non-22 K GH isoforms is poorly understood, but they may represent a spectrum of agonists or antagonists of the GH receptor.
Growth hormone secretagogues
Until 1977 it was thought that growth hormone release was only controlled by two hypothalamic mechanisms: stimulated by GH-releasing hormone (GHRH) and inhibited by somatostatin. Additionally, they observed that opiate peptides and analogues (like morphine) that are well established to release hGH in vivo had no activity on pituitary cells in vitro, indicating that these compounds have an hypothalamic site of action while the Met5-enkephalin appeared to act directly in the pituitary
Based on the structure of these new GH-releasing compounds that Bowers and coworkers identified, as well as the subsequent modelling studies, other compounds were developed leading to the nowadays known growth hormone secretagogue (GHS) family, which involves molecules that are apparently totally unrelated from a structural perspective; peptidic as GH-releasing peptide 6 (GHRP-6) or hexarelin and non-peptidic like the L-692,429 or the potent MK-0677.
All of them share the capability to release growth hormone from the cultured pituitary cells . Subsequently, researchers investigated the mechanisms of GHS action observing that, while GHRH induced the hGH secretion throughout an increase of cAMP, the newly developed secretagogues triggered the intracellular Ca2+ levels in order to achieve the hGH releasing activity.
Two different hGH secretion pathways in a somatotroph cell (graphic on the left). While the GHRH/Somatostatin way promotes the hGH secretion via the modulation of cAMP, GHS acts through the mobilization of intracellular calcium via the GHS-R1a. At this point, the fact that some synthetic compounds were able to promote the secretion of the growth hormone from the somatotroph cel s using the calcium mobilization entailed the presence of an hitherto unknown specific receptor, different from the GHRH-R, in these cel s and, in assumption that it was not an orphan receptor, an endogenous ligand for this new receptor should exist.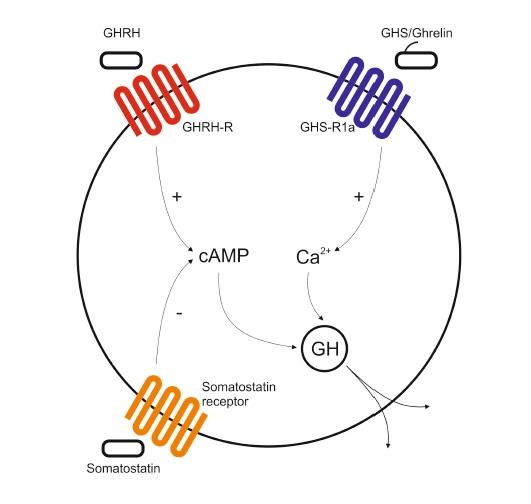
Growth hormone secretagogue receptor
In 1996, the receptor for the second GH-releasing mechanism was cloned. In their study the authors concluded that two different classes of the GHS receptor are encoded by the same gene. These two classes of the growth hormone receptor were named GHS-R1a and GHS-R1b. The GHS-R1a variant appeared to be a 366 amino acid polypeptide composed of 7 transmembrane domains while the 1b variant was a 289 amino acid long polypeptide with only five predicted transmembrane domains. Both variants of the same GHS receptor belong to the family of G protein-coupled receptors. The researchers also showed that the 1a variant is active under the secretagogue addition (MK-0677, GHRP-6 ) but not the 1b variant. For the latter until this day no function has been identified. Finally, it was also corroborated that the 1a receptor is only activated by growth hormone secretagogues but not GHRH, confirming the new growth hormone secretion pathway. GHS-R1a is mainly expressed in the pituitary gland but is also expressed, at a lower level, in a few other tissues such as: thyroid, pancreas, spleen, myocardium, and adrenal gland. On the other hand, the 1b variant is widespread, entailing a yet unknown physiological significance.
Endogenous GHS: Ghrelin
It was not until 1999 that the endogenous ligand for the GHS-R1a receptor was discovered in the rat stomach. Using a stable CHO cell line, expressing the GHS-R1a, and monitoring the intracellular calcium levels when a rat tissue extract was added to the media, the authors observed that the higher mobilization of calcium was found in the stomach sample. Purifying this extract they finally isolated a 28 amino-acid long peptide that was able to provoke the calcium mobilization on its own and they named this peptide “ghrelin”. The etymology is also unique, because “ghre” is the root of the word “grow”, but ghrelin evidently also connotes GH release. Moreover, other studies elucidate that ghrelin is mainly produced in the stomach, but it has also been found in other tissues.
Ghrelin resulted to be a 28 amino-acid long and since it acts as the endogenous ligand for the ghrelin receptor the GHS-R1a receptor was renamed GRLN receptor.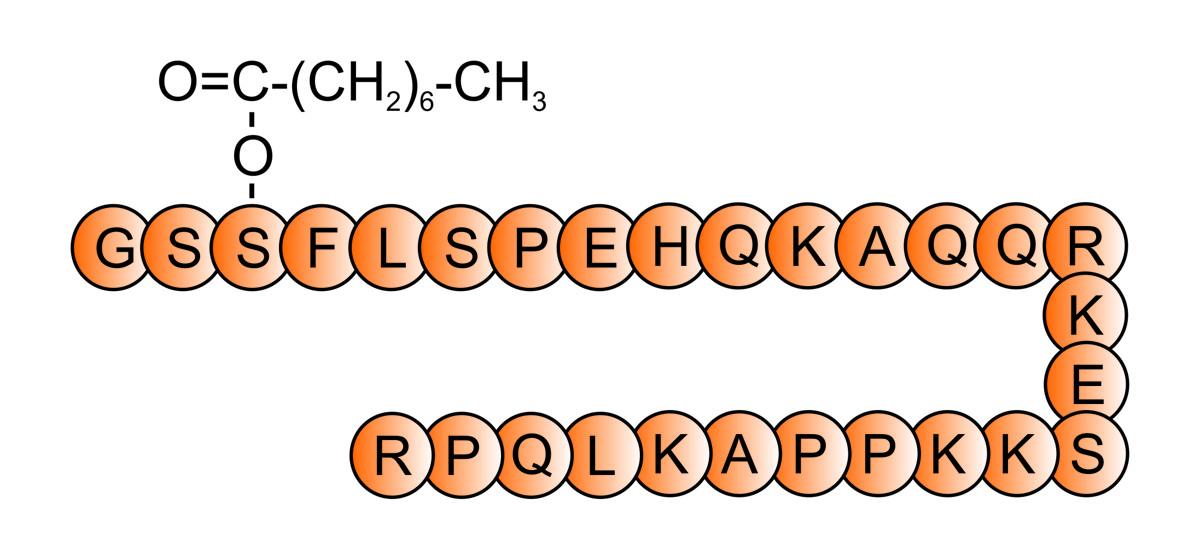
Biological functions of ghrelin
Alongside the hGH releasing activity, ghrelin resulted to perform several other functions. A notable function of ghrelin in the hypothalamus is its appetite-stimulating effect. In animals and in humans, ghrelin administration increases appetite, stimulates food intake and body weight gain. Ghrelin stimulates appetite by acting on the hypothalamic arcuate nucleus, a region known to control food intake. Furthermore, circulating ghrelin levels are increased by fasting and decreased by feeding, suggesting a role in meal initiation. Apart from regulating food consumption, ghrelin is somehow involved in lipid metabolism.
Some publications conclude that after binding of ghrelin in the hypothalamus, ghrelin receptor activates AMP-activated protein kinase (AMPK), and then it suppresses lipid synthesis but, on the other hand, other publications show that chronic delivery of ghrelin leads to body weight gain in rodents, not only through an increased appetite but also by promoting fat storage in white adipose tissue. Numerous studies in the last decade suggest that ghrelin has an important role in regulating β-cells function and glucose homeostasis . Ghrelin is also expressed in islet α- and ε-cel s in the pancreas, where it has also been shown to stimulate insulin secretion. Ghrelin also modulates gastric acid secretion and stimulates gastric motility by inducing the migrating motor complex and accelerating gastric voiding. Moreover, ghrelin exerts a gastroprotective effect against stress, ethanol, and cysteamine-induced ulcers. Furthermore, ghrelin has also diverse cardiovascular effects. It inhibits apoptosis of cardiomyocytes and endothelial cells in vitro. Ghrelin might counterbalance inflammation of the cardiovascular system by inhibiting the nuclear factor kappa-light-chain-enhancer of B cells (NF-kB) activation in human endothelial cells. Administration of ghrelin decreases mean arterial pressure without changing the heart rate and it improves cardiac contractility and left ventricular function in chronic heart failure and reduces infarct size.
Synthetic growth hormone secretagogues
Structures of some growth hormone secretagogues (GHS) can be observed below. ( MF, molecular formula; MM, molecular mass).
Encouraged by the potential of ghrelin as a therapeutic target, pharmaceutical companies are developing and testing both peptide and non-peptide compounds with pro- or anti-ghrelin activity. However, due to the multifunctional nature of ghrelin, both agonists and antagonists carry considerable potential to cause undesirable effects independent from the targeted primary indication. Interestingly, the concern of the pharmaceutical industry in these secretagogue compounds started prior to the discovery of ghrelin or its natural receptor. Seven years after the discovery of a met-enkephalin with in vitro growth hormone secretion effect, Bowers and coworkers presented GHRP-6, a hexapeptide that was able to elicit a dose-dependent release of hGH in vitro and in vivo in a variety of animal species that was accompanied by an increase of body weight [24;25]. Some years later, two analogues of GHRP-6 were developed , which were slightly more potent than GHRP-6 in vitro and threefold more effective in vivo. These new compounds, GHRP-1 and GHRP-2, conserve four of the six amino acid of GHRP-6. With the publication of GHRP-6, Mediolanum Farmaceutici synthesized Hexarelin that presented an enhanced potency and chemical stability with lower toxicity than GHRP-6. Hexarelin resulted to contain a methylation in one of the amino acids of GHRP-6. In a parallel way, Merck screened its compound library for GH releasing stimuli and some non-peptidic benzolactam molecules displayed a modest GHS activity. Developing these hits further finally the compound named as L-163 191 and posteriorly renamed as MK-0677 or Ibutamoren was synthesized. It was this compound that enabled the characterization and cloning of the GHS-R1a by introducing a radioisotopic label (32S) in the structure. MK-0677 resulted to be a high-affinity compound, unaffected by peptidases and presented and excellent bioavailability in canine. Apart from anything else, Merck synthesized ghrelin analogues with the aim of establishing the structural elements necessary to conserve its biological activity. Once the binding and activation of the GHS-R1a mechanism was elucidated, other pharmaceutical industries started their investigation in order to obtain a ghrelin analogue, antagonist or reverse agonist. Already in 2007, more than 50 ghrelin analogues with promising clinical capabilities were reported and this number keeps growing steadily. Nowadays, only GHRP-2 is commercially available (Kaken Pharmaceutical, Tokyo, Japan) but, may others compounds are being evaluated in human studies indicating that is only matter of time to have a diverse range of GHS available.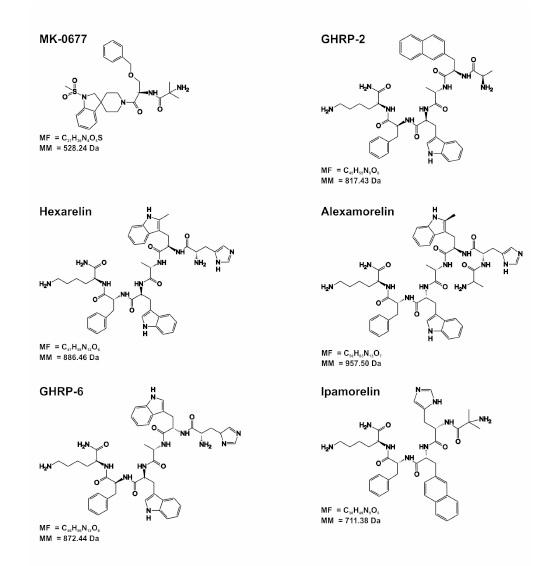
rhGH and GHS for doping purposes
Already in the ancient Olympic games, athletes have experimented with natural or synthetic compounds in order to improve their results. Records of special diets studied by athletes are reported to be as early as 668 B but with the advent of modern pharmacology in the 19th century, many athletes began to experiment with cocktails of synthetic drugs to improve strength and/or overcome fatigue. Growth hormone extraction and purification from human pituitary glands in 1956 lead to the discovery of its anabolic effects. It was shown to promote growth in hypopituitary animals and was soon used to treat children with hypopituitaris. The first record of hGH misuse by athletes was in 1982, when Dan Duchaine, considered an expert bodybuilder, published the book “Underground Steroid handbook”. In this book Duchaine explains the hGH benefits for athletes who claimed for an extra muscle gain. Cadaveric hGH was the only source of the hormone until the appearance in 1987 of the recombinant version of growth hormone (rhGH). The relationship of cadaveric hGH and Creutzfelt-Jacob disease led to its withdrawal from the market in 1985 although supplies of pituitary-derived hGH continue to be available on the black market. Thus, when rhGH appeared, it made the usage of this hormone easier and safer. The most famous case of rhGH abuse in professional athletics came to light in 1988 following Ben Jonson’s amazing win in the 100 m final at the Olympic Games in Seoul. His was subsequently disqualified when stanazolol was detected in his urine and he finally admitted that he had taken rhGH in addition to anabolic steroids. Although initially advocated for strength disciplines, endurance athletes are also attracted to rhGH’s lipolytic action and reduced fat mass and in 1988 a large quantity of rhGH was found in a team car at the Tour de France. Furthermore, the rhGH black market is no longer limited to elite athletes as nowadays a simple Internet search brings hundreds of sites where nutritional supplements containing claimed hGH (however often fake) could be bought. Nowadays in certain parts of the world (Australia) GHS are extremely popular.
Effect of ghrelin and anamorelin (ONO-7643)
Many patients with advanced stage malignancies often develop cachexia. This multifactorial syndrome is associated with both metabolic and endocrine-related effects. Cachexiais characterized by a ≥5 % weight loss, and other symptoms including loss of appetite, energy, muscle mass/strength, and functional performance. Cachexia can adversely affect a patients’ quality of life, their response to therapy, and their survival.
Ghrelin is a 28-amino acid peptide which acts as the endogenous ligand for the ghrelin receptor (GRLN receptor, formally known as GHS-R1a) . Administration of ghrelin to animals and humans has been shown to stimulate gastric acid secretion and motility, increase food intake and appetite leading to weight gain, promote anabolic activity, and inhibit production of pro-inflammatory cytokines, and thus may provide a viable target for cancer-related cachexia. Ghrelin activity is thought to be mediated by both growth hormone (GH)-dependent and GH-independent mechanisms. However, the short half-life (∼30 min), and parenteral administration requirement of ghrelin has limited its clinical usefulness, and interest has switched to the development of orally available ghrelin mimetics. One of these, anamorelin (ONO-7643, formally known as RC-1291), is a GRLN receptor agonist currently in development for the treatment of non-small-cell lung cancer (NSCLC)-related anorexia and cachexia. Through its ghrelin and GH-releasing activity, anamorelin has both orexigenic and anabolic properties. Anamorelin has been investigated in healthy volunteers and in cancer patients, where it was associated with significant increases in body weight (BW), total and lean body mass, and handgrip strength and improvements in patient-reported symptoms/quality of life assessments. These clinical studies have also shown increases in plasma levels of GH, insulin-like growth factor-1 (IGF-1), and insulin-like growth factor-binding protein 3 (IGFBP-3).
Importantly, neither ghrelin nor anamorelin promoted tumor growth in this murine xenograft model.
Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Northrup et all 2013
- Login to post comments


